Friday 30 August 2019
Lupine Publishers: Lupine Publishers | The Use of Epidural Anaesthesi...
Lupine Publishers: Lupine Publishers | The Use of Epidural Anaesthesi...: Lupine Publishers | The Journal of Veterinary Science Abstract General anaesthesia is an essential component of modern m...
Thursday 29 August 2019
Lupine Publishers: Change of Evaporations Leads to Climate Change
Lupine Publishers: Change of Evaporations Leads to Climate Change: Lupine Publishers- Environmental and Soil Science Short Communication The basis of all floods and droughts is water, its e...
Wednesday 28 August 2019
Lupine Publishers: Soil Texture of Nesting Sites and Breeding Populat...
Lupine Publishers: Soil Texture of Nesting Sites and Breeding Populat...: Lupine Publishers- Environmental and Soil Science Abstract Investigating the breeding population and soil texture of breeding ...
Tuesday 27 August 2019
Lupine Publishers: Lupine Publishers| Some Aspects of Red Special Win...
Lupine Publishers: Lupine Publishers| Some Aspects of Red Special Win...: Lupine Publishers- Environmental and Soil Science Abstract Due to increasing environmental radionuclide background, the scien...
Monday 26 August 2019
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...
Lupine Publishers: Lupine Publishers | Gastrointestinal Parasites Fou...: Lupine Publishers | Journal of Veterinary Science Abstract This paper is the first part of a three (3) part series of review...
Saturday 24 August 2019
Lupine Publishers: Lupine Publihsers | The Development Fortified Pan ...
Lupine Publishers: Lupine Publihsers | The Development Fortified Pan ...: Lupine Publishers | Journal of Veterinary Science Abstract The main objective of the research is to develop pan bread nutri...
Friday 23 August 2019
Lupine Publishers: Lupine Publishers | Use of Dietary Yeast and its P...
Lupine Publishers: Lupine Publishers | Use of Dietary Yeast and its P...: Lupine Publishers | Journal of Veterinary Science Abstract All around the world, sheeps and goats play an important role in ...
Thursday 22 August 2019
Lupine Publishers: Major Issues Related to Women Health, Social, Cult...
Lupine Publishers: Major Issues Related to Women Health, Social, Cult...: Journal of Gynaecology | Lupine Publishers Abstract Present article sketches out major issues related to health, social...
Wednesday 21 August 2019
Lupine Publishers: Optimization of Chitosan+Activated Carbon Nanocomp...
Lupine Publishers: Optimization of Chitosan+Activated Carbon Nanocomp...: Journal of Chemical Sciences | Lupine Publishers Abstract First, the minimum energy (geometry optimization DFT-DMol3) i...
Tuesday 20 August 2019
Lupine Publishers | Synthesis of Some Piperidine, Pyrmidine and Diazipine Compounds Containing Furyl Derivatives
Lupine Publishers | Journal of Nanomedicine
Abstract
Introduction
Experimental
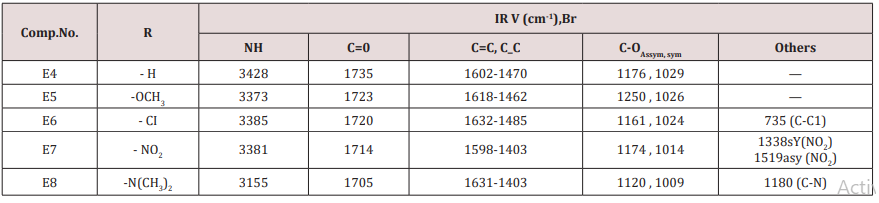
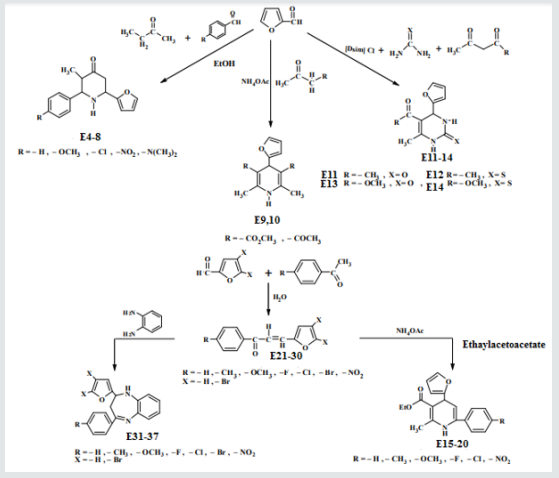
Synthesis of 2-(furan-2-yl)-6-aryl Piperidine-4-one(E4-8)
A mixture of 0.01mol. of ammonium acetate in 30ml. ethanol was mixed with 0.05mol. of benzaldehyde, 0.05mol. of furfural and 0.025mol. of 2-butanon.The mixture was refluxed for 2h, cooled and left for 24 h. at room temperature after that 15ml. of conc. HCl was then added, 20ml. of acetone. The suspended solution was treated ammonia solution then with water. The sold product filtered off and crystallized from ethanol physical and IR spectral data are presented in (Tables 1).Synthesis of some 1,4-dihydro Pyridine Derivatives (E9,10)
A mixture of (0.96g, 0.01mol.) furfural, 0.02mol. of (methyl aceto acetate or acetyl), 0.013mol. of ammonium acetate and 25 ml. of water. The reaction mixture was stirred at 70o C for 90 min. After that ethanol was added. The precipitated compound was filtered off, dried and crystallized from ethanol, physics and IR spectral data were as follows: compound 6, mp. 188-190o C , yield 74% as white crystals, compound 10 has mp. 177-179o C, yield 62% as yellow crystals . The IR spectra of those two compounds showed the following main absorption bands: 3346-3448cm-1,1705,1697,1653 and 1192, 1099,1219,1122.Synthesis of some 1,4-dihydro Pyrimidine Derivatives (E11-14)
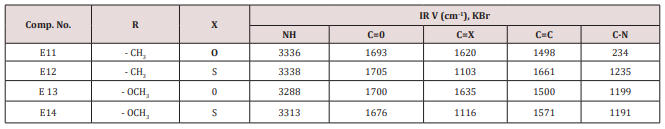
A mixture of furfural (2.4g, 0.025mol.), Urea or thiourea (0.033mol.) and acetyl acetone or methyl acetoacetate ( 0.12 g., 0.004mol.) of compound (2) was refluxed on 90o C for 20 min. using oil bath, cooled, water was added to precipitate the product which was filtered off and crystallized from ethanol , physical and IR spectral data were shown in (Table 2).Synthesis of 2-Methyl-4-(furan -2yl)-6-substituted phenyl-1,4- dihydro-3-pyridine carboxylate (E15-20)
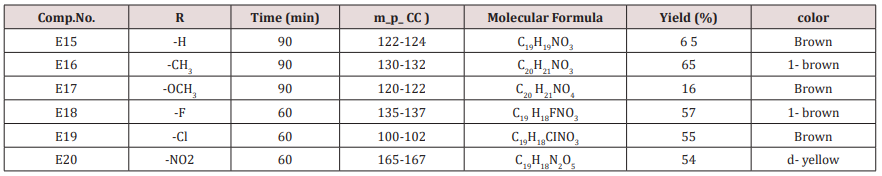
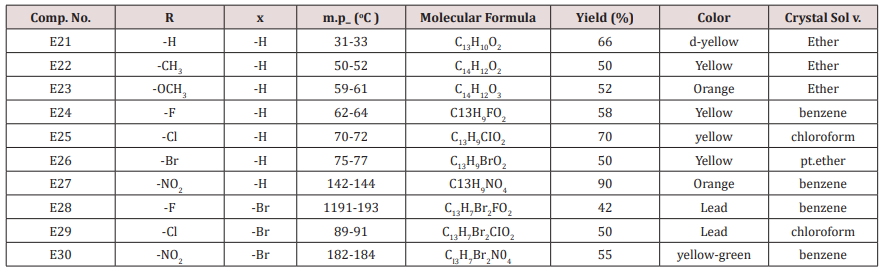
Ethyl acetoacetate (0.001mol. and compounds E25-31) each 0.01mol. and 0.01mol. Ammonium acetate were mixed with compounds (3), (0.05g.) in 5ml. of H2 O. The final mixture was refluxed with stirring for the indicated time (Table 5), cooled, 25mol. of dichloromethane was then added , filtered. The filtrate was evaporated under reduced pressure. The solid product was recrystallized from ethanol, physical and IR spectral data were shown in (Tables 3).Synthesis of 1-(4-substituted phenyl)-3-(4,5-dibrono furan-2-yl or furan-2yl)-2-propene-1-one(E21-30)
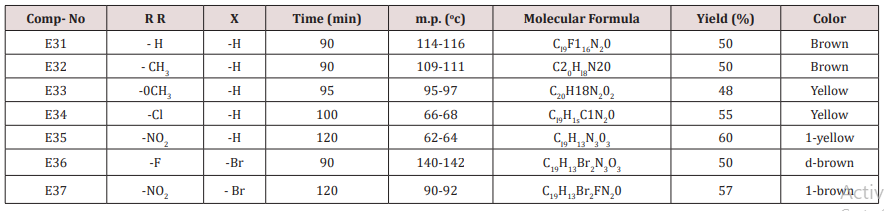
Furfural or 4,5-dibromo furfural (0.034mol.) and (0.034mol.) of acetophenone or its substitutes were mixed together and stirred at 5-15o C for 10 min., sodium hydroxide 10% was then added drop wise. After complete addition, the mixture was stirred for further 3-4 hours. The reaction mixture was left for 24 hours. The solid ppt. was washed with cold water, physical and IR spectral data are presented in (Tables 4).Synthesis of 2-(Furan-2-yl)-4-phenyl -2,3-dihydro -1H-benzo[b] ,[1,4]-diazepines(E31-37)
Compounds (E13-23), 0.01mol., ortho phenylene diamine (0.01mol.) were mixed together, cellulose- sulfonic acid (0.01 mol.) was then added . The reaction mixture was refluxed at 80o C for a given time Tables (10) the reaction was monitored by TLC after completion it was cooled and extracted with 3x10 of ether. The combined ether was dried, and the solvent was evaporated under reduced pressure. The solid product was crystallized from etherethyl acetate, Physical and spectral data are presented in (Table 5).Results and Discussion
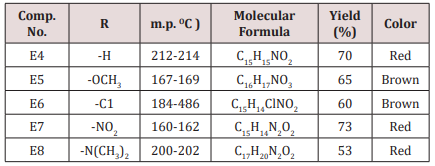
Compound (E11-14)
This series of compounds were prepared by condensation of compounds (E4-8) with Urea, thiourea , acetyl acetone or methyl aceto acetate using [Dsim] as catalyst(scheme1). The synthesized compound was characterized by IR (Table 7).The first two compounds of this series (E11-12) were characterized by 1HNMR as follows: For E11 compound (s) signal at 9.30ppm. Belongs to NH proton, (d) at 7.50ppm. For proton at position 5 of furan ring, (t) signal at 6.35ppm. fpr proton of position 4 of furan ring, (d) signal at 6.10ppm. For proton of position 3 of furan ring, (d) at 5.3ppm. For the Piperidine proton, (5) of 3.37ppm. belongs to C=OCH3 protons and (d) at 2.23ppm. for CH3 protons. While compound E12 characterized by the following resonating signals ʃppm.: 10.31, 9.7 (s) belong to NH proton, (s) at 7.58 for position (5) proton of furan ring, (t) at 6.38 for position (4) proton, (d) at 6.17 for position (3) proton of furan ring, (d) at 5.36 for the Piperidine proton, (s) at 3.30 assigned to COCH3 and (s) at 2.20 to CH3 equivalent to 3H.
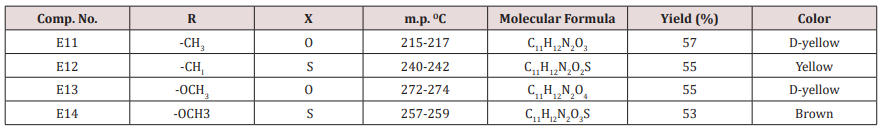
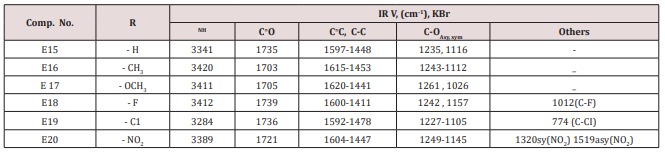
Compounds (E15-20)
This series of compounds were prepared from the condensation of the corresponding chalcones with ethyl aceto acetate, ammonium acetate using cellulose sulfonic acid as catalyst. These compounds were characterized by the following IR absorption bands; see (Table 8). This Table revealed the presence of NH absorbed at 3284-3420cm-1 for NH, 1703-1739cm-1 for C=C while C-O sym and assym appeared at 1026-1261cm-1, see Table 8. 1HNMR spectrum of compound E16 gave the following resonating signals ʃppm.: multiplet(m) signal at 7.42,7.59,7.65,7.77,7.82 and 8.07 related to 5 position protons of furan and the aromatic ring protons, NH proton respectively. Triplet signal at 6.40 belongs to proton of furan ring, (d) at 6.33 for position 3 proton of furan ring, (d) at 5.00 for proton 5 of furan ring, (d) at 4.63 for Piperidine proton of position 4, (q) at 4.18 for C2 of ester moiety, (s) at 3.88 for CH3 of the substituted at 2 position of Piperidine, (s) at 2.50 for CH3 of ester, 1.94 as double belongs to CH3 of ester see.Compounds (E<21-30)
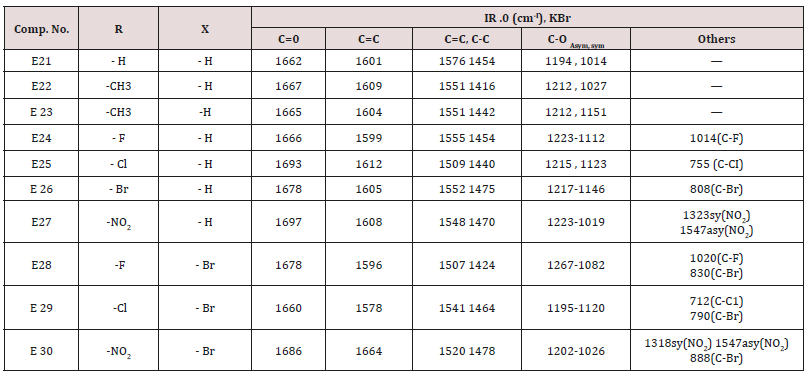
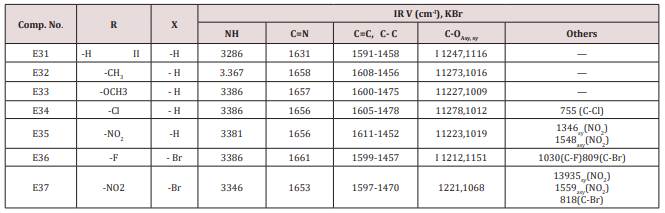
This series of chalcone compounds were prepared from the condensation of 4,5-di bromo furfural with some acetophenones (Scheme 1). The synthesized compounds were characterized by IR, Table. This Table revealed the presence of C=O stretching vibration at 1660-1697cm-1, C=C Aliphatic appeared at 1578-1664cm-1, while the aromatic C=C absorbed at 1416-1576cm-1 together with C-O sym and assym as indicated in (Table 9). These chalcone compounds were used as starting materials for the preparation of diazipine compounds by the condensation with ortho phenylene diamine using sulfamic acid to increase the positive character of the carbonyl chalcones. The final(diazipin) compounds were characterized by the following IR bands cm-1: 3188-3346 for NH, 1631-1661 for C=N, 1452-1611 for Aromatic C=C stretching, C-O sym and assym. of 1009-1253, see (Table 10 ). As a representative sample of this series compound E36 was studied by 1HNMR . The spectrum showed the following resonating signals: (m) signal at 6.96,7.36 and 7.81 for furan ring protons in which aryl and phenyl ring protons appeared within the same range, (s) signal at 6.26 for proton of position 2 of azipine ring, (s) signal at 3.33 for NH, singlet (s) signal at 2.50 for CH3 which is consider with DMSO signal, (d) at 2.24 belongs CH2 of a zipine ring protons.
Conclusion
Acknowledgments
For more Lupine
Publishers Open Access Journals Please visit our website https://lupinepublishersgroup.com/
For more Journal
of Nanomedicine articles Please Click Here: https://lupinepublishers.com/nano-science-nano-technology-journal/
To Know more about Open
access Publishers, click on Lupine
Publishers.
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
Wednesday 14 August 2019
Lupine Publishers: Lupine Publishers - Quora
Lupine Publishers: Lupine Publishers - Quora: Lupine Publishers is a premier, peer-reviewed, multidisciplinary, open access, scientific Publisher that aims to publish original work of im...
Friday 9 August 2019
Lupine Publishers: Lupine Publishers | Anti-Synepthelichorial Placent...
Lupine Publishers: Lupine Publishers | Anti-Synepthelichorial Placent...: Lupine Publishers | Journal of Veterinary Science Hens were immunized with a macerate of goat and sheep placentomes. Immunizations w...
Tuesday 6 August 2019
Lupine Publishers: Experimenting with New Crops at the Peri-Urban Fri...
Lupine Publishers: Experimenting with New Crops at the Peri-Urban Fri...: Agriculture open access journals | Lupine Publishers Farming at the Peri-Urban Fringe As the world’s human population ...
Friday 2 August 2019
Lupine Publishers: Lupine Publishers | Survey on Pathological Lesion ...
Lupine Publishers: Lupine Publishers | Survey on Pathological Lesion ...: Lupine Publishers | Journal of Veterinary Science Abstract A cross-sectional study was conducted from October 2016 to July...
Subscribe to:
Posts (Atom)
Lupine Publishers: Lupine Publishers | Post Endodontic Pain Reduction...
Lupine Publishers: Lupine Publishers | Post Endodontic Pain Reduction... : Lupine Publishers | Journal of Otolaryngology Research Impact Fac...
-
Flaky Academic Journals: Lupine Publishers "craves to select scientific con... : Lupine Publishers describes itself (on its website, 2/...
-
Lupine Publishers: Lupine Publishers | Is There Any Relation Between ... : Lupine Publishers | Open access journal of Complimentary & Al...
-
Lupine Publishers: Lupine Publishers | The Prevention and Treatment o... : Lupine Publishers | Open Access Journal of Complementary & A...