Lupine Publishers | Journal of Nanomedicine
Abstract
Introduction
Experimental
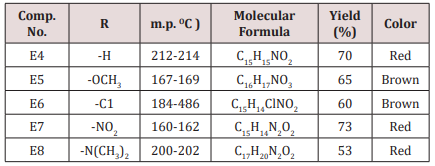
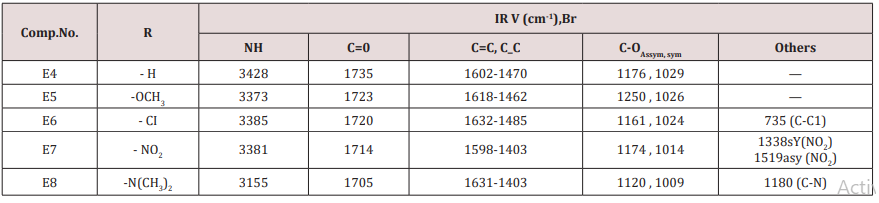
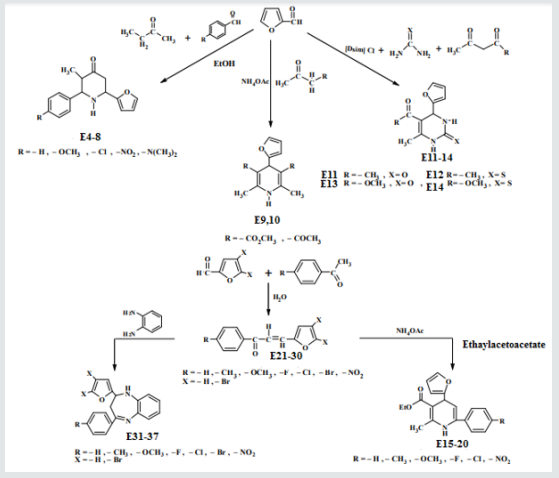
Synthesis of 2-(furan-2-yl)-6-aryl Piperidine-4-one(E4-8)
A mixture of 0.01mol. of ammonium acetate in 30ml. ethanol was mixed with 0.05mol. of benzaldehyde, 0.05mol. of furfural and 0.025mol. of 2-butanon.The mixture was refluxed for 2h, cooled and left for 24 h. at room temperature after that 15ml. of conc. HCl was then added, 20ml. of acetone. The suspended solution was treated ammonia solution then with water. The sold product filtered off and crystallized from ethanol physical and IR spectral data are presented in (Tables 1).Synthesis of some 1,4-dihydro Pyridine Derivatives (E9,10)
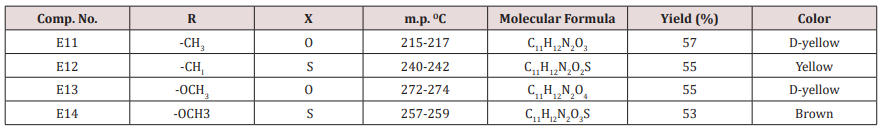
A mixture of (0.96g, 0.01mol.) furfural, 0.02mol. of (methyl aceto acetate or acetyl), 0.013mol. of ammonium acetate and 25 ml. of water. The reaction mixture was stirred at 70o C for 90 min. After that ethanol was added. The precipitated compound was filtered off, dried and crystallized from ethanol, physics and IR spectral data were as follows: compound 6, mp. 188-190o C , yield 74% as white crystals, compound 10 has mp. 177-179o C, yield 62% as yellow crystals . The IR spectra of those two compounds showed the following main absorption bands: 3346-3448cm-1,1705,1697,1653 and 1192, 1099,1219,1122.Synthesis of some 1,4-dihydro Pyrimidine Derivatives (E11-14)
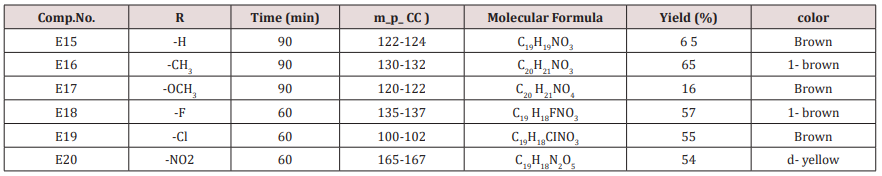
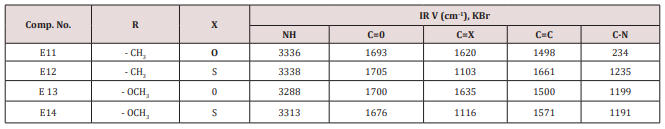
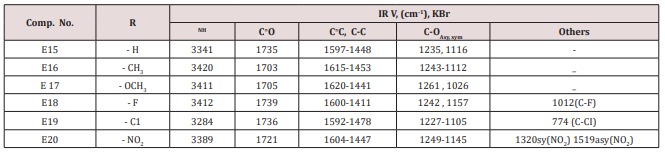
A mixture of furfural (2.4g, 0.025mol.), Urea or thiourea (0.033mol.) and acetyl acetone or methyl acetoacetate ( 0.12 g., 0.004mol.) of compound (2) was refluxed on 90o C for 20 min. using oil bath, cooled, water was added to precipitate the product which was filtered off and crystallized from ethanol , physical and IR spectral data were shown in (Table 2).Synthesis of 2-Methyl-4-(furan -2yl)-6-substituted phenyl-1,4- dihydro-3-pyridine carboxylate (E15-20)
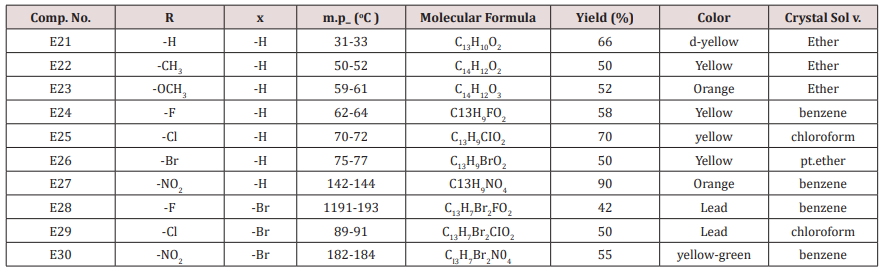
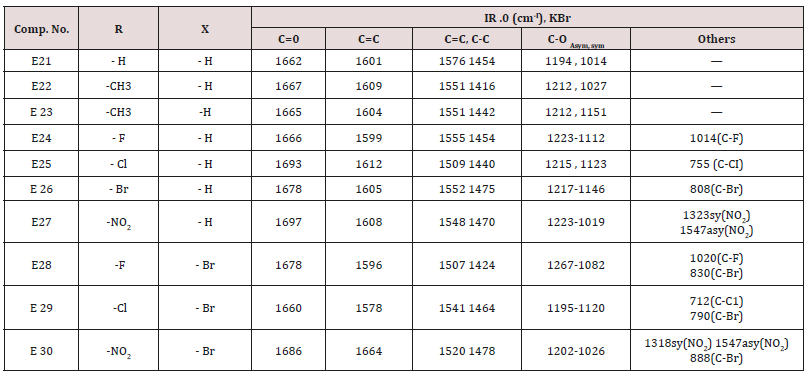
Ethyl acetoacetate (0.001mol. and compounds E25-31) each 0.01mol. and 0.01mol. Ammonium acetate were mixed with compounds (3), (0.05g.) in 5ml. of H2 O. The final mixture was refluxed with stirring for the indicated time (Table 5), cooled, 25mol. of dichloromethane was then added , filtered. The filtrate was evaporated under reduced pressure. The solid product was recrystallized from ethanol, physical and IR spectral data were shown in (Tables 3).Synthesis of 1-(4-substituted phenyl)-3-(4,5-dibrono furan-2-yl or furan-2yl)-2-propene-1-one(E21-30)
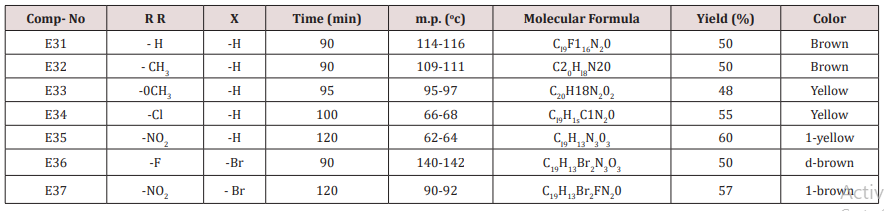
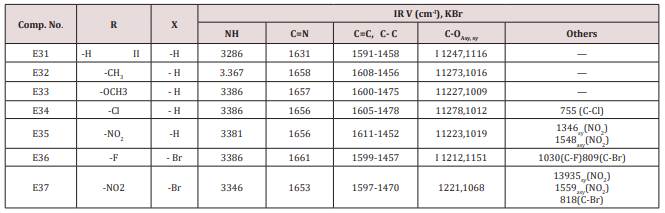
Furfural or 4,5-dibromo furfural (0.034mol.) and (0.034mol.) of acetophenone or its substitutes were mixed together and stirred at 5-15o C for 10 min., sodium hydroxide 10% was then added drop wise. After complete addition, the mixture was stirred for further 3-4 hours. The reaction mixture was left for 24 hours. The solid ppt. was washed with cold water, physical and IR spectral data are presented in (Tables 4).Synthesis of 2-(Furan-2-yl)-4-phenyl -2,3-dihydro -1H-benzo[b] ,[1,4]-diazepines(E31-37)
Compounds (E13-23), 0.01mol., ortho phenylene diamine (0.01mol.) were mixed together, cellulose- sulfonic acid (0.01 mol.) was then added . The reaction mixture was refluxed at 80o C for a given time Tables (10) the reaction was monitored by TLC after completion it was cooled and extracted with 3x10 of ether. The combined ether was dried, and the solvent was evaporated under reduced pressure. The solid product was crystallized from etherethyl acetate, Physical and spectral data are presented in (Table 5).Results and Discussion
Compound (E11-14)
This series of compounds were prepared by condensation of compounds (E4-8) with Urea, thiourea , acetyl acetone or methyl aceto acetate using [Dsim] as catalyst(scheme1). The synthesized compound was characterized by IR (Table 7).The first two compounds of this series (E11-12) were characterized by 1HNMR as follows: For E11 compound (s) signal at 9.30ppm. Belongs to NH proton, (d) at 7.50ppm. For proton at position 5 of furan ring, (t) signal at 6.35ppm. fpr proton of position 4 of furan ring, (d) signal at 6.10ppm. For proton of position 3 of furan ring, (d) at 5.3ppm. For the Piperidine proton, (5) of 3.37ppm. belongs to C=OCH3 protons and (d) at 2.23ppm. for CH3 protons. While compound E12 characterized by the following resonating signals ʃppm.: 10.31, 9.7 (s) belong to NH proton, (s) at 7.58 for position (5) proton of furan ring, (t) at 6.38 for position (4) proton, (d) at 6.17 for position (3) proton of furan ring, (d) at 5.36 for the Piperidine proton, (s) at 3.30 assigned to COCH3 and (s) at 2.20 to CH3 equivalent to 3H.
Compounds (E15-20)
This series of compounds were prepared from the condensation of the corresponding chalcones with ethyl aceto acetate, ammonium acetate using cellulose sulfonic acid as catalyst. These compounds were characterized by the following IR absorption bands; see (Table 8). This Table revealed the presence of NH absorbed at 3284-3420cm-1 for NH, 1703-1739cm-1 for C=C while C-O sym and assym appeared at 1026-1261cm-1, see Table 8. 1HNMR spectrum of compound E16 gave the following resonating signals ʃppm.: multiplet(m) signal at 7.42,7.59,7.65,7.77,7.82 and 8.07 related to 5 position protons of furan and the aromatic ring protons, NH proton respectively. Triplet signal at 6.40 belongs to proton of furan ring, (d) at 6.33 for position 3 proton of furan ring, (d) at 5.00 for proton 5 of furan ring, (d) at 4.63 for Piperidine proton of position 4, (q) at 4.18 for C2 of ester moiety, (s) at 3.88 for CH3 of the substituted at 2 position of Piperidine, (s) at 2.50 for CH3 of ester, 1.94 as double belongs to CH3 of ester see.Compounds (E<21-30)
This series of chalcone compounds were prepared from the condensation of 4,5-di bromo furfural with some acetophenones (Scheme 1). The synthesized compounds were characterized by IR, Table. This Table revealed the presence of C=O stretching vibration at 1660-1697cm-1, C=C Aliphatic appeared at 1578-1664cm-1, while the aromatic C=C absorbed at 1416-1576cm-1 together with C-O sym and assym as indicated in (Table 9). These chalcone compounds were used as starting materials for the preparation of diazipine compounds by the condensation with ortho phenylene diamine using sulfamic acid to increase the positive character of the carbonyl chalcones. The final(diazipin) compounds were characterized by the following IR bands cm-1: 3188-3346 for NH, 1631-1661 for C=N, 1452-1611 for Aromatic C=C stretching, C-O sym and assym. of 1009-1253, see (Table 10 ). As a representative sample of this series compound E36 was studied by 1HNMR . The spectrum showed the following resonating signals: (m) signal at 6.96,7.36 and 7.81 for furan ring protons in which aryl and phenyl ring protons appeared within the same range, (s) signal at 6.26 for proton of position 2 of azipine ring, (s) signal at 3.33 for NH, singlet (s) signal at 2.50 for CH3 which is consider with DMSO signal, (d) at 2.24 belongs CH2 of a zipine ring protons.
Conclusion
Acknowledgments
For more Lupine
Publishers Open Access Journals Please visit our website https://lupinepublishersgroup.com/
For more Journal
of Nanomedicine articles Please Click Here: https://lupinepublishers.com/nano-science-nano-technology-journal/
To Know more about Open
access Publishers, click on Lupine
Publishers.
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
No comments:
Post a Comment